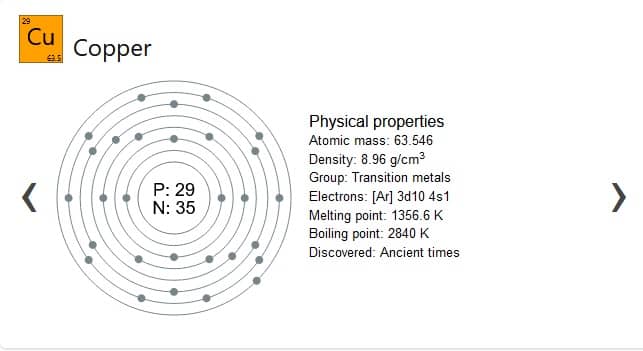
Why is copper a great conductor? Second most abundant metal on earth so it is cheap. Copper is a fairly common element, with an estimated concentration of 50–70 ppm (0.005–0.007 percent) in earth’s crust (1 kg of copper per 15–20 tons of crustal rock). It is malleable and can be easily bent or coiled.
Melting point: 1,984°F (1,085°C)
- Discovered: 9000 BC
- Symbol: Cu
- Electron configuration: [Ar] 3d10 4s1
- Boiling point: 4,644°F (2,562°C)
The electron configuration for copper is 1s2 2s2 2p6 3s2 3p6 4s1 3d10. Copper has one electron in the 4th orbit or valance orbit so it is more loosely held than other elements. The conductive orbit and valance orbit cross over so it is easier for the free electron in 4h orbit to break away.